return to Contents
original text of the thesis:
Population dynamics of the Gyrinid beetle Gyrinus marinus Gyll (Coleoptera)
With special reference to its dispersal activities (1987)
CHAPTER IV REPRODUCTION
SUMMARY
Between 1974 and 1983 data concerning egg production and recruitment were obtained by sampling populations of the whirligig water beetle Gyrinus marinus Gyll. All females
reproduce, but they differ in the number of eggs laid per oviposition and in the frequency of
oviposition. There are two reproducing generations a year, one in spring (April-June) and one in summer (July-August). Females emerging in July and August reproduce immediately after emergence, but at a lower level than the females of the spring generation. In June the egg production of spring females also decreases. No significant differences in egg production occur between populations in different sites or in different years. Individual properties and circumstances of the females probably have a distinct influence on variation in egg production. A female probably oviposites and is fertilized at least once a week. Without fertilization egg production and the viability of the eggs decrease after two weeks. On the average, 60 per cent of the eggs hatch.
Development from egg to teneral takes about 10 weeks in spring and autumn and about six weeks in summer. The hatching and development of eggs laid in April is probably retarded until the half of May. Variation in the length of the developmental period will cause variation in recruitment and in the number of hibernating beetles. Variation in egg-viability and in larval and/or pupal development are probably of equal importance to the variation in the numbers of tenerals emerging. The variation in egg production is of no importance.
Recruitment (number of tenerals/female) of the generations reproducing in spring and in summer are independent of each other. Between years the rate of recruitment differs significantly, and probably depends more on environmental conditions such as weather than egg production seems to do.
1. INTRODUCTION
1.1. Reproduction consists of (a) the reproductive activities of females and males (fertilization,
egg production and egg laying), and (b) development of the eggs until the emergence of tenerals (recruitment). It is frequently supposed that a population reacts to changing circumstances (such as food shortage) by adjusting its egg production, particularly if density-dependent processes have been assumed, which are usually thought to have a regulating effect on population size (e.g. Baars and van Dijk 1984). By means of key factor analyses (Varley and Gradwell 1960, Podoler and Rogers 1975) a number of case studies have shown that the mortality of a stage in the development between egg production and the
emergence of adults is a key factor in the population dynamics of the species (e.g. review in Podoler and Rogers 1975).
1.2. We have carried out measurements and experiments on the re-production of whirligig beetles (Gyrinus marinus Gyll.), to obtain estimates of the relationships between egg production, development and final recruitment. These measurements and experiments are part of a comprehensive study of the population dynamics of whirligig beetles which also includes survival and dispersal.
1.3. Reproduction of whirligig beetles starts with the laying of eggs under water. After hatching, the larvae grow up under water and pupate outside the water. Each stage in the development from ovocyt to mature adult beetle has its specific growth rate and its own survival chance. The influence of each stage upon the ultimate variation in recruitment is of special interest. For example, if egg production has the greatest influence, the living conditions of the females will probably have a significant influence upon the size of recruitment. But if the conditions for the larvae under water provide the most important factor for the variation in recruitment it may be fairly unimportant to recruitment what happens to the females. We therefore give special attention to variation in egg production and to that in the emergence of tenerals. A full description of reproduction will embrace (a) information about reproductive activities both of females (number of females reproducing, number of ovipositions, number of
eggs per oviposition, individual differences) and of males (frequency of fertilizations, influence of fertilization upon egg viability), and (b) information about development from egg to adult (proportion of eggs which hatch, time of development, variation in survival) and about recruitment (number of tenerals per female, per generation, and per month).
2. METHODS
2.1. As gyrinid beetles live on the water surface of small water bodies they are convenient subjects for poppulation dynamic field studies. Most of the data were collected from ten populations in the main study-area in the northern part of the Netherlands near Groningen. These were supplemented with data from other populations within a radius of about 20 km around Groningen. The populations will be indicated by location-symbols and year: for example, Zp-76 and Wn-83 concern populations Zp in 1976 and Wn in 1983 (for the meaning of the location-symbols see Fig III-3).
2.2. In the field the eggs are laid on submersed water plants, but the number of eggs produced per female can easily be measured by putting single females in tubes or petri-dishes with moistened toilet paper for 24 hours. The ripe eggs will then be laid in rows on the paper. When the paper on which the eggs are laid is kept moist, the eyes of the embryos become visible through the thin egg scale and it is possible to ascertain the number of viable eggs after five days at room temperature.
Young females start oviposition about 10 days after emergence. Unless otherwise stated we used only females with hardened elytra (females older than two weeks) in the experiments.
From population Zp-76 10 to 20 females of each sample taken in April until June were kept in formalin and later dissected to count the number of eggs in the ovaries.
By repeatedly measuring the egg production of the same individually-marked females from populations Zp-76 and Mp-83 the egg production of individual females was estimated during the reproduction season. (The technique of individual marking is described in chapter VI Dispersal by flight). In this way it was possible to estimate the rate of egg production in the ovaries, the frequency of ovipositions and the frequency of fertilizations per female.
2.3. If a female was fertilized before she was captured a spermatophore was also left on the paper. After 20 June 1983 all males were removed from population Mp-83 to trace the influence of both the frequency of copulations and of the duration of the effect of a single fertilization on egg production and on the viability of the eggs laid.
2.4. Recruitment is estimated by the percentage of 'very soft' beetles (tenerals) in the samples.
After a beetle has emerged, it takes one to several weeks before the hardening of the cuticula,
especially of the elytra, is completed. Until five to seven days after emergence the beetles can be distinguished from previously emerged tenerals.
2.5. Time will be indicated by numbered weeks. In each year the same seven days bear the same number. In this way data of different years are directly comparable (i.e. 1 April always is the last day of week 13, etc.).
2.6. Variation in the data is expressed as the variation coefficient (v.c.=standard deviation/
mean value). Variation coeffecients in time sequences (data of the same population in different years) will be indicated as v.c.t , and in space sequences (data of different populations in the
same period) by v.c.p.
2.7. Statistical tests used are all reffered to Sachs 1983. Unfortunitely different authors use
different symbols in the same tests. The tests and the symbols used in this paper are as follows:
t-test, parameter t; Mann-Whitney U-test: U, for large samples normally distributed parameter z; Kruskall-Wallis H-test: H, z; correlation test: r or z; Spearman correlationtest: r, z; Chi-square test: X2; Anovar: F; Wilcoxon paired signed-rank test: R, z; Fisher-test: z. If possible the normally distributed parameter z is used, because of the comparability of different tests and its independence of the size of the samples.
3. RESULTS
A. Egg production
Egg production will first be considered at population level and then at individual level (per
female). Finally the influence of fertilization upon egg production will be discussed.
A.1. Egg production per generation.
A.1.1. Egg production starts after hibernation in April and continues until mid-August. About
the end of June the first young beetles emerge. These beetles start to reproduce within two weeks. The offspring of this generation emerges from early September until hibernation at the end of October. The successive reproducing generations only partly overlap (about 25 per cent in summer and about 2 per cent in spring).
As long as a female is alive she produces eggs, until about the last week of August (week 35).
Females caught on the first day of activity already have many developing ovocytes. It takes several weeks before all females are reproducing, and the number of ripe eggs in the overies increases until the second half of May (weeks 19 - 22, see Fig. IV-1).
During the period for which both measurements are available the number of eggs laid per female is correlated with the number of eggs in the ovaries (1976, weeks 16 - 24, comparing the regression coefficients of the time sequences of eggs laid and eggs in ovaries: t=0.84, df=404, P > 0.05). But the number of eggs laid per week is significantly higher than the weekly number of eggs in the ovaries (Mann-Whitney U-tests per week: all tests P<0.02). After week 22 (early June) the number of eggs laid decreases significantly with time (Spearman test r = -0.819, n=41, P<0.001). Females of the summer generation start oviposition about ten days after emergence, but their egg production is lower than that of females of the spring generation. (Mann-Whitney U-tests: see Table IV-1). It is striking that the decrease in egg production of the spring generation begins as early as the second week of June (week 23), i.e. about six weeks before the first oviposition of the summer generation
(Spearman test on mean numbers of eggs laid per female: weeks 22 - 27, n=13, r=-0.698, P<0.02).
The decrease of egg production of the spring generation is due both to a decrease in the number of eggs laid per oviposition and to a decrease in the fraction of females that oviposites (Table IV-1; Spearman tests: weeks 22 -27, eggs/ovip.: n=347, z=3.017, P<0.005; % fem/ovip.: n=12, r=0.612, P<0.05).
A.1.2. The differences in egg production between the generations in spring and in summer may be the result of a greater heterogeneity in the ages of the individuals of the summer generation. We have seen above that in springtime it takes some weeks before the females come fully to reproduction. The same may be true for the young females of the summer generation. Young beetles can be roughly divided into five age classes depending on the degree of hardening of the elytra. A number of experiments were carried out to relate egg production to age (Table IV-2). A significant correlation is found between hardness of the elytra and the proportion of reproducing females (Spearman test: n=23, r=-0.50, P<0.01), but between hardness and the amount of egg production per female no correlation can be stated (n=23,r=-0.18, P>0.10).
Another reason for the decreasing egg production from June to the end of the egg production period is probably to be found in the decreasing fraction of females that has been fertilized (i.e. fraction of females with spermatophores, see Fig. IV-2 and A.3).
A.1.3. The variation in numbers of eggs laid in summer is significantly higher than in springtime (U-test on v.c.-values in Table IV-1: z=3.58, P<0.002), due to the decreasing mean number of eggs laid per oviposition from June onwards. Comparing different populations in the same week of the same year (variation in space) gives a mean v.c.p= 0.17 (st.dev. = 0.08, n=6), comparing populations in the same week of different years gives a mean v.c.t= 0.14 (st.dev. = 0.08, n=12). Analyses of variance show no significant influence of time or space on the variation in egg production (Anovar tests: 10 x P>0.10,
2 x P<0.02), except a significant influence of time between week 16 and week 25, when egg
production is building up (Anovar: 4 x P<0.002, 1 x P<0.05, 1 x P>0.10). Apparently the number of eggs laid per female is less dependent on circumstances that differ specifically from place to place or from year to year (such as the availability of food) than on factors connected with the individual beetles. Alternatively, such circumstances may not have fluctuated in space or time in a way that significantly influences egg production in a population. A logical next step is therefore to consider the egg production inside the generation i.e. per individual female.
A.2. Egg production per individual female.
A.2.1. The egg production of individual females in the course of time under field conditions
can be studied by frequently recapturing individually marked females and testing oviposition capability and egg production on moist paper (see Methods). The tests were carried out in 1983 with females of the Mp-83 population.
A.2.2. There were no females that did not reproduce at all. If tested in two successive weeks every female laid eggs at least once, except during the first two tests in April. If tested in four consecutive weeks 16 out of 17 females laid eggs three or four times. About 80 per cent of the females in a sample laid eggs, so a female seems to have ripe eggs most of the time (Table III-3). The successive tests on 8 and 13 June show that development of a new set of ripe eggs in the ovaries can be accomplished in five days. This indicates that oviposition in the field may occur more frequently than our sampling, i.e. more frequently than weekly. Indeed many developing ovocytes and nearly ripe eggs were found in females that were dissected immediately after egg laying (Fig. IV-2).
From recaptures of newly hatched females it appears that 9 out of 10 females can lay eggs within 10 days after emerging (cf A.3.4.)
A.2.3. Individual females show significant differences in egg production, i.e. some females lay more eggs per oviposition than others (Anovar: df=48,55; F=2.22; P=0.01). From the Anovar test it appears that about 80 per cent of the total variation in egg production is due to variation-in-time of individuals.
In A.1 it was shown that egg production in spring is higher than in summer, but also that from June onwards egg production decreases. One possible reason for this (cf. A.1) could be the increasing age of the females. Females that are not recaptured again can be considered to have died, either from old age or from an accident (e.g. predation). Generally, the last time a female was recaptured she laid less eggs than she did at previous captures (captures 2 May to 20 June; Wilcoxon test: n=17, T=21, P<0.01). Significantly more females did not lay eggs at their last capture than at their previous captures (X2test: df=1, X2= 5.31, P<0.025). In Table III-3 some results concerning repeated oviposition of individual females are reviewed.
A.3. Effects of fertilization on egg production and on viability of eggs.
A.3.1. In June and July 1983 in several Populations a mean viability of eggs was found of 59.5
per cent (st.dev. 35.54, v.c. = 0.60).
A.3.2. In 67.3 per cent (152 out of 226) of the oviposition tests a spermatophore was left with the eggs on the paper; it was less frequently found without eggs (12 out of 161 spermatophore records: 7.5 %). A female is apparently fertilized several times during the reproduction period at about the same frequency as she is laying eggs. But since the percentage of females captured with a spermatophore decreased from week 22 onwards (Spearman test: r=-0.585, n=10, P<=0.05, see Fig IV-3), the fraction of fertilized females is lower in the summer generation than in the spring generation (U-test: n1=n2=15, U=26.5, z=3.568, P<0.001). The situation is comparable with that of the numbers of eggs laid per oviposition (Fig IV-1). The decrease in the percentage of spermatophores starts before the first individuals of the new generation emerge. Probably the capacity for spermatophore production decreases with increasing age of the males, similar to the decreasing egg production of females at old age. Newly emerged males may have to spend some time to built up their spermatophore production (cf A.3.4). Frequent fertilization may be important for egg production. In experiments with pairs of carabid beetles in petri dishes the females were fertilized several times a week. In the absence of fertilization the egg production and the viability of the eggs decrease (van Dijk pers.comm.). The same probably applies to gyrinid beetles.
A.3.3. To test the role of fertilization all males were removed on 20 June from the Mp-83 population and in the following weeks the reproduction of the females was recorded. Newly emerged males were removed, but we could not prevent a number of females from being fertilized in the second week of July (the somewhat better results at 18 July may be due to that, see Table IV-4). During the first two weeks the removal of the males influenced neither egg production nor the viability of the eggs (Table IV-4). After three weeks the percentage of reproducing females had not yet changed, but the number of eggs laid per female decreased significantly (t-test: df=136, t=2.335, P<0.025), as did the viability of the eggs (Table IV-4: % of females with viable eggs: X2-test df = 3, X2= 30.21,P<0.001; Table IV-3: % viable eggs: X2-test df=3, X2= 106.1, P<0.001). A second indication for the importance of fertilization for egg production and development is found in the significant difference in viability between eggs of females with and those without a spermatophore (Fisher test (a) on % of females with viable eggs: n=126, z=6.416, P<0.001; (b) on % of viable eggs: n=43, z=1.87, P<0.05).
A.3.4. In this experiment it also appeared that the egg production of young females starts irrespective of fertilization. But to maintain egg production as well as a high fraction of viable
eggs frequent fertilizations are necessary.
On 11 July we captured eleven females with a spermatophore. These females must have been fertilized by newly emerged males. From our sampling we know that first males emerged about 1 July. Hence, within about ten days after emerging males are capable of producing a spermatophore. (The increase of the number of reproducing males is possibly also indicated by the - not significant - temporary increase of fertilized females between week 26-31 in Fig.IV-3.)
Moreover, among the egg-laying females there were also young females that had emerged about 1 July, i.e. females are also able to start reproduction within about 10 days after emerging.
B. The recruitment
The development from egg to teneral (recently emerged beetles) takes several weeks and can be divided into several stages, each with its own survival rate, time of development and variation. We were not able to collect data about larval development under water or about pupation. But from the egg production and the number of beetles that have emerged weekly, some estimates can be made about survival rates during development.
First, the proportion of tenerals in the total population, and the time required for development
from egg to adult will be considered. Then we will be in a position to estimate the numbers of emerging beetles per week. From these analyses it is possible to estimate both the development rate during the whole season, the recruitment per generation, per female and per oviposition, and the survival rate from egg until emergence.
B.1. The proportion of tenerals in the samples
B.1.1. Recently emerged beetles (tenerals) are easy to recognize by their very soft cuticula. The process of hardening takes about ten or more days; tenerals in a sample can be considered as not older than about one week. By measuring the proportion of tenerals in the samples the weekly emergence of new beetles can be followed. Emergence begins in all years in the 25th week (about 20 June), reaching a maximum in week 27 (first week of July). The different times at which egg production begins from year to year have little influence on the first date that tenerals start emerging (Fig IV-4). Females and males emerge in equal percentages. Until about week 33 (mid-August) the proportion of emerged tenerals decreases, and then increases again due to the emergence of the autumn generation. A second maximum is reached in weeks 36 - 38 (early September). Emergence then decreases again until it is terminated by hibernation at the end of October (about week 43). No tenerals are found at the begining of
the next spring. The time of emergence of the summer generation is more stable from year to year then that of the autumn generation (Fig IV-4).
B.2. The rate of development from egg to teneral
B.2.1. In order to relate the number of tenerals emerged in a given period to the number of
parent-females in a previous period of egg laying, the time required for development from egg to teneral must be known.
B.2.2. Each year the first eggs are laid in weeks 15 - 17 and the first tenerals in great numbers
are captured in week 26. The time required for development is thus nine to eleven weeks. However, in summer development is more rapid. Egg production of the summer generation starts at about week 28 and the first tenerals are found in week 33 - 35 (Fig IV-4), that is, development is completed in five to seven weeks.
B.2.3. It is well known that the time required for the development of insects depends on temperature. Blunck (1914, 1924), studying the rate of development of eggs and larvae of Dytiscus marginalis L.(a waterbeetle living in the same waters as Gyrinus marinus), found that the hatching of eggs and the growth of larvae takes much more time when the temperature is below 15oC than when it is higher than this. The rate of development does not change with variation in temperature above 15oC. Van Dijk (1979b) found threshold temperatures of about 10oC for egg-laying of the carabid beetle Pterostichus versicolor Sturm, and a strong delay in hatching and the growth of larvae at low temperatures (van Dijk, pers. comm.). Ringelberg (1976) described the temperature during the year for different Dutch pools. As the pools in our study area are not deeper than 1 to 2 meters we may assume from Ringelberg's data that water temperatures at the bottom (where Gyrinus-larvae live) exceed 15oC by the middle of May and are below 15oC again by the beginning of October.
B.2.4. If the temperature regime for Gyrinus development is comparable with that of Dytiscus, the hatching of eggs laid in April and at the beginning of May would be retarded for several weeks whereas in summer eggs would hatch within one week. First instar larvae would have a low rate of development until the temperature rises above the threshold value. If the threshold temperature is exceeded about mid-May, the time required for development in springtime would be about 5 or 6 weeks, like in summer. As a consequence of retarded hatching and of the different rates of development, both the tenerals from eggs laid in April and those from eggs in May should emerge from the end of June through the first half of July (weeks 26 - 29). Egg production ends at week 35 (last week of August) and the number of tenerals decreases after about week 40 (when water temperature is below 15oC).
B.3. The number of emerging tenerals
B.3.1. The number of emerged tenerals per week (Yi) can be estimated from the percentage of tenerals per sample (zi) when the total number of beetles (Ai) is known. However, direct estimates of the total numbers of beetles are not reliable enough to give reliable values for Yi. The total number of beetles and the number of emerged beetles per week are estimated by an iterative method, in combination with weekly estimates of the survival chance and of the population size by capture-recapture methods (Chapter V Survival of adults). The results are estimated for 1000 beetles in week 14 at the beginning of the season. See the Appendix for an explanation of the method.
B.3.2. Recruitment is usually estimated per generation as the mean number of tenerals per female, by dividing the total number of tenerals by the total number of females. This may be valid for reproduction in spring, although mortality is ignored: when the last tenerals are emerging many of the first ones are already dead. Information about possible differences in reproduction succes in different months is also lost. But for reproduction in summer the total number of tenerals cannot be related to the total number of females, because the number of reproducing females during summer increases by newly emerged tenerals. It therefore seems better to relate recruitment and the number of reproducing females seperately for each month for both generations, since the same procedure must be followed to permit comparison between the generations.
B.3.3. Because of the changing development rates it is not possible to relate the number of
reproducing females in one period (week or month) directly to the number of beetles emerging after a given number of weeks. We may assume, however, that there is a relation between the number of eggs laid in a given month and the number of tenerals that originates from that month of egg-laying. On this assumption, the number of tenerals of one generation per month of egg production is proportional to the number of eggs laid in the corresponding month. The number of eggs laid in a month depends on the number of eggs per oviposition (m, cf. Table IV-1) and on the number of females (Fi) that oviposit each week in the relevant month, i.e. it depends on the sum (Sf) of that number of females: Sf = 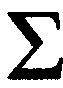 (Fi). A measure for the number of eggs laid in a month is found by Se = m*Sf. The total number of eggs laid per generation (SSe) is found by adding the Se-values of the months: SSe =
(Fi). A measure for the number of eggs laid in a month is found by Se = m*Sf. The total number of eggs laid per generation (SSe) is found by adding the Se-values of the months: SSe = 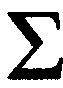 (Se). The total number of tenerals per generation is found by Y =
(Se). The total number of tenerals per generation is found by Y = 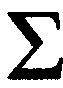 (Yi). The number of tenerals per month is estimated then as Sy = Y*(Se/SSe).
(Yi). The number of tenerals per month is estimated then as Sy = Y*(Se/SSe).
B.4. Recruitment per oviposition, per female and per generation
B.4.1. On the average half (47 %) of the recruitment of the spring generation originates from egg production in May, about 37 per cent from that in June and only 16 per cent can be attributed to egg production in April. Egg production in July accounts for 35 per cent of the recruitment in the autumn generation; 65 % derives from egg production in August. (All differences appear significant with Wilcoxon tests on n=14 populations, P<0.025; Table IV-5.A.)
B.4.2. Recruitment of different populations, of different generations, or in different years can
be compared at three levels: in relation to the numbers of females, of ovipositions, or of eggs.
The monthly sum (Sf) of the numbers of egg-laying females per week is a measure for the mean number of week-ovipositions in that month. Sy/Sf then gives the mean number of tenerals that result from one week of egg laying of a female (Table IV-5.B (Sy)). Because the monthly Sy-values are estimated proportionally with the mean monthly egg production, the proportion of the Sy/Sf values for the different months within the same generation mainly varies due to variation in survival and is hardly affected by variation in reproduction. Variation coefficients for the different months within the spring generation therefore show little variation (cf. Table IV-5.B (Sy/Sf)). In summer the variable influx of newly emerged females also has an effect on the variation of Sy/Sf.
On the average the egg production of a female during one week results in spring in 1.8, and in summer in 1.5 tenerals (i.e. per oviposition in case of one single oviposition per female per week). Differences between months are not significant (H-test, df=4, H=4.3, P>0.30).
If X is the number of ovipositions per female in one week (X=1 or X>1, see A.2.), then an average estimate of survival during the development from egg te teneral (i.e. of reproduction success can be found by relating Sy/Sf to the number of eggs laid per oviposition (m). A reproductive success of 0.08/X for the spring and of 0.05/X for the summer generation is thus found (Table IV-5.C (Sy/Sf)/m)), i.e. at best (if X=1, one oviposition /female/week) one of every 12.5 eggs in spring and one of every 20 eggs in summer develops into a teneral.
B.4.3. The number of females that oviposites in a month fluctuates depending on survival and in summer also on the influx of new females. Therefore, when recruitment is related to the number of egg-laying females in a given month, it must be related to the number of females that has oviposited at least once in the month concerned (nf, see B.3.1). The average estimated Sy/nf-values are given in Table IV-5.D (Sy/nf). In April, in July and in August on the average 3 tenerals emerge from the total monthly egg production per female; in May and June this is 8 tenerals per female (Wilcoxon test: significant difference between May or June and one of the
other months, n=14, T=4, P<0.01).
Although recruitment from spring- and from summer generation are about equal per oviposition, females reproducing in May and June produce more tenerals per month than females of the summer generation. This is due to the higher number of weeks during which a female will lay eggs in May and June than in summer (on the average 4.16 and 1.91 weeks respectively) as well as to the lower proportion of eggs that will develop into a teneral in summer than in spring.
B.4.4. There are significant differences between the years in regard to the recruitment of the
summer generation (both tenerals per oviposition and tenerals per female), but not of the spring generation (Anovar: df1= 3, df2= 11; spring: F = 2.58, P>0.10; summer: F=6.93, P< 0.05).
No relation is found between the recruitment (either per female, per oviposition or per generation) of the spring generation and that of the summer generation (n=14, r=0.12, P>0.05), i.e. the recruitments of each reproducing generation in a year apparently varies independently of the other.
4. DISCUSSION
1. Variation in egg production
1.1 After egg production has reached its maximum in mid-May it decreases again in June both in terms of the number of eggs laid per oviposition and in terms of the proportion of females that oviposites. This decrease continues during the reproduction of the summer generation in July and August.
1.2. There are some indications that this decreasing reproductive effort is due to some factor
connected with the beetles themselves, apart from possible external factors such as food shortage. A probable negative relation was found between the number of eggs laid per oviposition and the increasing age of the female (cf. 3.A.2). The reproduction of young females, that are just starting, is also low. Furthermore egg-production appeares dependent on the frequency of fertilization. As the frequency with which a spermatophore is deposited decreases from May to August, it may be that a decrease in the frequency of copulations causes the decrease in egg-production.
1.3. An Anovar-test on the sequences of ovipositions of individual females indicates that most
variation in egg production is due to variation in time per female, i.e. to the temporary circumstances of life of each female (luck in getting food, males or shelter, influences of weather and water quality, etc.).
1.4. Another indication of the importance of the life histories of individual females may be that
the standard deviations of egg production per oviposition do not decrease when the mean number of eggs laid decreases (Table IV-1). If we suppose that the upper limit of the number of eggs laid per oviposition depends on the number of ovarioles of a female, then, when the mean number of eggs laid per oviposition decreases, due to accidental factors, the upper limit will not change, leading to an unchanged standard deviation. The variation in egg production between populations and between generations is smaller than between the females in a population (coefficient of variation v.c.=0.17 and v.c.=0.14 versus v.c.=0.39). If some external factor, like food supply, quality of food and temperature were important for variation in egg production per population, a greater variation in time and place should be expected than was actually found. Apparently, the different habitats are rather similar concerning these kinds of features. But, although great numbers of insects may be drowned daily at the water surface (Norlin 1964, 1967) - potential food for whirligig beetles - great differences between individuals may occur in the quantity and quality of the food taken. Some studies support the
hypothesis that predators (at least insects) may struggle with food-shortage in spite of plenty of preys being available (van Dijk 1986, White 1978, Dempster and Pollard 1981).
2. Variation in recruitment
2.1. Variation between estimates can be due both to the process studied and to errors of estimation. Egg production was measured directly from ovipositions, but recruitment was estimated indirectly from other estimates like survival chance, number of females, number of ovipositions, etc. The values given for variation in recruitment are therefore less reliable than those concerning egg production.
2.2. Each of the developmental stages from egg to emerged tenerals has its own variation. The
importance of each stage for the variation in final recruitment can be analysed in a manner analogous to the key-factor analysis of Varley and Gradwell (Varley and Gradwell 1960, Southwood 1978). The first stage is that of egg-development and oviposition (k0). A maximum potential natality of about 38 eggs per oviposition was found in spring and of about 32 eggs per oviposition in summer. On the average 32 eggs are laid per oviposition in spring and 25 in summer (cf. Table IV-1, variation coefficient between populations: v.c.t= 0.14). About 55 per cent of the eggs hatch, with a variation coefficient of v.c = 0.34 (k1). No estimates are available for the larval and pupal stages, but it was estimated that 1.8 tenerals emerge from the egg-production in one week in spring (v.c. = 0.54) and 1.5 in summer (v.c = 0.32) (k2).
2.3. A simple stochastic simulation model was constructed to trace the influence of the variation in survival in each stage upon ultimate recruitment. In this model the stages concerned are described by normal frequency distributions defined by the estimated mean and standard deviation of their survival rates. For each generation values for the survival rates of the concerned stages are drawn at random from their normal distributions. The model was run for 100 spring generations and for 100 summer generations separately (Fig IV-5) and Table IV-6. There is no single distinct key-factor. Variation in egg production does not play any part. A regression analysis on the k-values (cf. Podoler and Rogers 1975) confirms the impression from Fig IV-5 that variation in k2 (i.e. in larval and pupal development) contributes most to the changes in K in spring and variation in k1(viability of eggs) does so in summer (see Table IV-6 ).
3. Variation in egg production and in recruitment compared
3.1. We have suggested that egg production is possibly more dependent on the capacities and
vicissitudes of individual females than on conditions at the population and/or habitat level. Variation in egg production between populations is small; all our field data can be considered as samples from one statistical population.
3.2. In contrast, recruitment appeares to be significantly dependent on conditions at population or habitat level. If density-dependent processes do occur, they probably work during the development between oviposition and the emergence of tenerals more than during egg production and egg laying. The same is suggested by Heessen and Brunsting (1981) for carabid beetles, and by Istock (1966) for Dineutus beetles (American gyrinids). The absence of any correlation between the recruitments of the spring and summer generations gives no indication of any density-dependent process that influences the amount of recruitment.
4. Influence of the time required for development
4.1. Reproduction is generally seen as a numerical process in which a given number of females lays a given number of eggs, which provides a given number of young adults as recruitment. In theoretical analysis, especially in computer simulation models, the role of the time taken for development from egg to adult is often disregarded. But when population dynamics are described in separate processes for reproduction, survival and dispersal, taking account of the time occupied by each process, it becomes clear that time can be an essential parameter. For example, the longer the development of the larvae, the later the number of reproducing females in the second generation will increase, the smaller the number of tenerals that emerge in autumn, and so the smaller the number of adults that hibernate. The total recruitment in a whole year will decrease with 65 to 80 per cent if the time required for development is increased only two weeks, respectively from 6 to 8 and from 8 to 10 weeks. Furthermore, survival during development is probably lower when development takes more time.
Given that hibernation begins at the end of October, the end of the period of reproduction and the time required for development are about optimal.
4.2. A maximum in emergence occurs shortly before hibernation, providing a large number of beetles to enter hibernation. Egg production in September would be too late for larval and pupal development to be completed before winter. A shorter developmental period (which is probably not possible anyway) would lead to too early emergence and thus to a smaller number of beetles in hibernation.
5. Influence of the frequency of fertilization
5.1. When fertilization fails for several weeks, egg production decreases and most of the eggs laid are not viable. Apart from its influence within the population, the frequency of fertilization may also have important consequences for the egg production of females that emigrate by flight. Dispersal by flight occurs in low numbers, also during the reproduction period, but more by males than by females (Chapter VI Dispersal by flight). Since the influence of a single fertilization ceases after about two weeks, it may be functional that males show a greater flight activity than females; especially in newly founded populations, females must be soon accompanied by males.
return to Contents
REFERENCES
Baars MA, THS van Dijk (1984) Population dynamics of two carabid beetles at a Dutch heathland II. Egg production and survival in relation to density. J Anim Ecol 53:389-400
Blunck H (1914) Die Entwicklung des Dytiscus marginalis L. vom Ei bis zur Imago. 1 Teil. Das Embryonalleben. Z wiss Zool 111:76-151
Blunck H (1924) Die Entwicklung des Dytiscus marginalis L. vom Ei bis zur Imago. 2 Teil. Die Metamorphose (B. Das Larven und das Puppenleben). Z wiss Zool 121:171-391
Boer PJ den (1981) On the Survival of Populations in a Heterogeneous and Variable Environment. Oecologia (Berl.) 50:39-53
Dempster IP, Pollard E (1981) Fluctuations in resource availability and insect populations.
Oecologia (Berl.) 50:412-416
Dijk ThS van (1979a) Reproduction of young and old females in two carabid beetles and the
relationship between the number of eggs in the ovaries and the number of eggs laid. Miscell Papers LH Wageningen 18:167-183
Dijk ThS van (1979b) On the relationship between reproduction, age and survival in two carabid beetles: Calathus melancephalus L. and Pterostichus coerulescens L. (Col., Carabidae). Oecologia 40:63-80
Dijk ThS van (1983) The influence of food and temperature on the amount of reproduction in
carabid beetles. How to translate the results of laboratory experiments into reality of the field?
Rep 4the Symp European Carabid. 1981
Dijk ThS van (1986) How to estimate the level of food availability in field populations of
carabid beetles. XVII Int Congr of Entomol Hamburg 1984 (in press)
Eijk RH van der (1983) Populations dynamics of gyrinid beetles I: Flight activity of Gyrinus
marinus Gyll. Oecologia (Berl.) 57:55-64
Eijk RH van der (1986) Populations dynamics of gyrinid beetles III: Survival. Oecologia
(Berl.) 69:41-46
Heessen HJL (1980) Egg production of Pterostichus oblongopunctatus (Fabr.) (Col.,Carab.) and Philonthus decorus (Grav.)(Col., Staph.). Neth J Zool 30:35-53
Heessen HJL, Brunsting AMH (1981) Mortality of larvae of Pterostichus oblongopunctatus (Fabr.) and Philonthus decorus (Grav.). Neth J Zool 31:729-745
Istock CA (1966) Distribution, coexistence and competition in whirligig beetles. Evolution 20:211-234
Murdoch WW (1966) Aspects of the population dynamics of some marsh Carabidae. J Anim Ecol 35:127-156
Norlin A (1964) The occurrence of terrestrial insects on the surface of two lakes in northern
Sweden. Rep Inst Freshwat Res Drottingholm 45:196-205
Norlin A (1967) Terrestrial insects in lake surfaces. Rep Inst Freshwat Res Drottingholm 47:40-55
Ochs G (1969) The ecology and ethology of whirligig beetles. Arch Hy-drobial Suppl 35(4):375-404
Podoler H, Rogers D (1975) A new method for identification of key factors from life-table data. J Anim Ecol 44:85-114
Reddingius J, Boer PJ den (1970) Simulation experiments illustrating of animal numbers by spreading of risk. Oecologia 15:245-258
Ringelberg J (1976) Aquatische oecologie in het bijzonder van het zoete water. Bohn, Scheltema & Holkema, Utrecht (in Dutch)
Southwood TRE (1978) Ecological methods (2nd ed.). Chapman and Hall, London
Sachs L (1982) Applied Statistics. A Handbook of Techniques. Springer, New York
Thiele HU (1977) Carabid beetles in thei ť�vironments. Springer Berlin, Heidelberg, New York
Varley GC, Gradwell GR (1960) Key factors in population studies. J Anim Ecol 29:399-401
White TCR (1978) The importance of a relative shortage of food in animal ecology. Oecologia (Berl.) 33:71-86
return to text (B.3 Number of emerging tenerals)
APPENDIX
Estimation of the number of emerging beetles (tenerals)
Example of the estimation of the rate of recruitment of a population.
Procedure: Fit the numbers estimated from field data concerning survival and recruitment to capture-recapture estimates of the population size, by adjusting either the values of survival or that of recruitment (depending on their reliability).
General assumptions (apart from those mentioned in text, B.3.1.):
- Emergence is sufficiently regular to be represented by a mathematical function.
- The survival of tenerals may be lower than that of beetles older than 2 weeks (importance of fouraging in the first week).
- Dispersal activities are too low to affect the numbers or the composition of the population.
Population size estimated by capture-recapture is callibrated at 1000 beetles in week 14 (see Fig. IV-6D):
In Table IV-7 the survival rates for males and females of the three generations in population Ks are given, together with the percentage of tenerals per sample followed by three adjusting runs using different survival rates per run. The consequences for the estimated population size are given in Fig IV-6 A-C.
Comments:
In the first run the proportion of tenerals in the not sampled weeks is interpolated according to the trends in the mean values of all populations in 1974 (Fig IV-6A).
In the second run survival values are decreased, tenerals have got a worse survival chance that other beetles, and the proportion of tenerals has been changed to achieve lower numbers and to bring the point of maximum population size forward in time (Fig IV-6B).
In the third run survival in summer is divided into a survival chance of 0.9 before week 30 and a survival chance of 0.85 from week 30 onwards. The proportion of tenerals in weeks 36-39 has also been changed (Fig IV-6C). Numbers estimated with these data fits the best with the estimated capture-recapture data of Fig. IV-6D.
return to text (B.3 Number of emerging tenerals)
return to Contents